 |
| Scientific Learning Class 10 Science Guide/Note. |
i. Investigate independent variables, dependent variables and control variables in scientific research and mention the importance of the control variable.
ii. Differentiate between fundamental and derived units.
iii. Investigate the fundamental units involved in different derived units.
iv. Mention and use the fundamental units in physical units in physical equation to examine their homogeneity.
SEE Class 10 Compulsory Science | All Units Notes:
- Unit-1 | Scientific Learning | Class 10 | Science | Guide.
- Unit-2 | Classifications of Living Beings | Class 10 | Science | Guide.
- Unit-3 | Honeybee | Class 10 | Science | Guide.
- Unit-4 | Heredity | Class 10 | Science | Guide.
- Unit-5 | Psychological structure and Life Process | Class 10 | Science | Guide.
- Unit-6 | Nature and Environment | Class 10 | Science | Guide.
- Unit-7 | Motion and Force | Class 10 | Science | Guide.
- Unit-8 | Pressure | Class 10 | Science | Guide.
- Unit-9 | Heat | Class 10 | Science | Guide.
- Unit-10 | Wave | Class 10 | Science | Guide.
- Unit-11 | Electricity and Magnetism | Class 10 | Science | Guide.
- Unit-12 | Universe | Class 10 | Science | Guide.
- Unit-13 | Information and Communication Technology (ICT) | Class 10 | Science | Guide.
- Unit-14 | Classification of Elements | Class 10 | Science | Guide.
- Unit-15 | Chemical Reaction | Class 10 | Science | Guide.
- Unit-16 | Gases | Class 10 | Science | Guide.
- Unit-17 | Metals and Non-Metals | Class 10 | Science | Guide.
- Unit-18 | Hydrocarbon and Its Compounds | Class 10 | Science | Guide.
- Unit-19 | Chemicals Used in Daily Life | Class 10 | Science | Guide.
View All SEE Class 10 Compulsory Science Notes:
SEE Class 10 Compulsory Science | All Units Notes and Question Papers Collection.
Scientific Learning
SEE Class 10 Science Notes.
? Test Your Concepts
1. Multiple Choice Questions (MCQs):
2. Differentiate between:
| Independent Variables | Dependent Variables |
|---|---|
| i. The variables that do not depend on other variables. | i. The variables that depend on other variables. |
| ii. Independent variables are controllable. | ii. Dependent variables are not controllable. |
| iii. Explain the cause and effect of changes in the variables. | iii. Explained by the independent variables. |
| iv. Independent variables are influenced by the researcher. | iv. Changes in response variables are directly caused by the changes in independent variables. |
| v. Independent variables are exposure variables that are used in the research. | v. Dependent variables are the outcome of the research. |
| Aspect | Independent Variable | Dependent Variable |
|---|---|---|
| Definition | A variable that is adjusted or modified. | Variable that depends on another for its value. |
| Role | Alters or influences the dependent variable. | Responds to changes in the relevant variable. |
| Symbol | Frequently shown in equations as x. | Frequently shown in equations as y. |
| Example | The amount of fertilizer applied is the independent variable when examining how fertilizer affects plant growth. | Plant growth is the dependent variable in a study on the impact of fertilizer. |
| Control | It can be manipulated or controlled by the researcher. | Typically, the researcher has less control over it. |
| Position of Axes in Graphs | Displayed using the x-axis. | Displayed on a y-axis plot. |
| Purpose in Equations | Examining the effect on the dependent variable is frequently the aim. | Finding its relationship to the independent variable is frequently the aim. |
| Fundamental Unit | Derived Unit |
|---|---|
| i. Basic units of measurement that are independent of others. | i. Units that are derived from fundamental units. |
| ii. Independent; not formed from other units. | ii. Dependent on fundamental unit. |
| iii. Defined directly by international standards. | iii. Formed by combining two or more fundamental units using mathematical operations. |
| iv. They are SI Base Units. | iv. They are SI Derived Units. |
| v. Examples: Meter (m), Kilogram (kg), Second (s), Ampere (A), Kelvin (K), Mole (mol), Candela (cd) | v. Examples: Newton (N), Joule (J), Watt (W), Pascal (Pa), Coulomb (C), etc. |
| Fundamental Units | Derived Units |
|---|---|
| i. Fundamentals units are those units which are independent of any other unit. | i. Derived units are the units that results from a mathematical combination of SI base units. |
| ii. Fundamental units cannot be further reduced to elementary level; in fact, these are elementary units. | ii. Derived units can be reduced to its elementary level, which are composed of fundamental units. |
| iii. Fundamental units can not be expressed in terms of derived units. | iii. Derived units can be expressed in terms of derived units. |
| iv. Only seven fundamental units exist in Metric System or SI system. | iv. There exist a large number of derived units in Metric System. |
| v. Examples: Length, Mass, Time, Temperature, Electric Current, Luminous Intensity, Amount of Substance. | v. Examples: Velocity, Acceleration, Momentum, Force, Density, Heat, Energy, Power, etc. |
| Fundamental Quantity | Derived Quantity |
|---|---|
| i. Basic physical quantities that are independent of others. | i. Quantities derived from fundamental quantities. |
| ii. Independent; not derived from other quantities. | ii. Dependent on fundamental quantities. |
| iii. Examples: Length, Mass, Time, Temperature, Electric Current, etc. | iii. Examples: Speed, Area, Volume, Density, Force, Pressure, etc. |
| iv. There are only 7 fundamental quantities exist. | iv. There are unlimited (numerous) derived quantities exist. |
| v. Have their own SI base units (e.g., meter, kilogram, second) | v. Expressed in terms of base units (e.g., m/s, kg/m³, N = kg·m/s²) |
| vi. Basic form (e.g., [L], [M], [T]) | vi. Combination of dimensions (e.g., [M¹L¹T⁻²] for force) |
3. Very short answer type questions:
a. What is scientific learning?
Answer:
b. What are various steps of scientific learning?
Answer:
c. What are control variables?
Answer:
Control variables are those variables that are maintained unchanged or constant in an experiment.
d. What are dependent and independent variables in research?
Answer:
e. Write clear examples of independent, dependent, and controlled variables.
Answer:
Example: In an experiment testing how sunlight affects plant growth:
Independent variable: Amount of sunlight.
Dependent variable: Plant height.
Controlled variables: Water, soil type, and plant species kept the same.
f. What are the independent, dependent, and controlled variables in an experiment investigating the reaction rate between calcium carbonate and dilute hydrochloric acid?
Answer:
Independent variable: Concentration of hydrochloric acid.
Dependent variable: Rate of reaction (e.g., amount of gas produced over time).
g. State the principle of homogeneity of equations.
Answer:
h. Which physical quantity is represented by kg/m³?
Answer:
i. If a zoologist is turning a bulb on and off to observe the movement of a cockroach, what would be considered the independent variable in this experiment?
Answer:
j. Define fundamental and derived units.
Answer:
k. Define a unit. Write its types.
Answer:
l. Name the fundamental units used in physics.
Answer:
Meter (m) – for length
Kilogram (kg) – for mass
Second (s) – for time
Ampere (A) – for electric current
Kelvin (K) – for temperature
Mole (mol) – for amount of substance
Candela (cd) – for luminous intensity
4. Short answer type questions.
a. What are variables in research? Explain with examples.
Answer:
Research Question: Does listening to music while studying affect memory recall?
i) Independent Variable: Listening to music (yes/no).
ii) Dependent Variable: Memory recall score.
iii) Controlled Variables: Study time, difficulty of material.
Research Question: In a study examining the effect of study time.
i) Independent Variable: the amount of the study time.
ii) Dependent Variable: the test scores.
b. Clarify different types of variables giving an example of experiment.
Answer:
ii) Dependent Variable: Reaction time (measured in milliseconds using a computer test).
c. What is the dimension of a physical quantity? Give an example.
Answer:
Example: Force
Mass → [M]
Acceleration →
So,
✅ Final Answer:
The dimension of force is .
d. Why is the independent variable referred to as the predictor variable, and the dependent variable as the responding variable?
Answer:
The independent variable is referred to as the predictor variable because it is used to forecast or predict the outcome of the dependent variable. The dependent variable is referred to as the responding variable because it is what changes or responds in reaction to changes in the independent variable.e. Why is the joule the SI unit of work and energy?
Answer:
- Work is the process of transferring energy.
- When work is done, energy is transferred or transformed.
f. Why is the unit of power referred to as a derived unit?
Answer:
| Summary | |||
|---|---|---|---|
| Quantity | Formula | Unit | Derived From |
| Power | Work ÷ Time | Watt (W) | kg·m²/s³ (derived from J/s) |
| Work | Force × Distance | Joule (J) | kg·m²/s² |
g. The unit of pressure is a derived unit, why?
Answer:
h. Why is it important to consider controlled variables in scientific research?
Answer:
i. Why is it not permissible to add or subtract physical quantities with different basic units?
Answer:
j. What is the unit of work? Explain whether the unit of work is a fundamental or derived unit?
Answer:
k. Why the unit of force is called a derived unit?
l. What is a derived unit? Give its two examples.
Answer:
m. Define measurement and unit.
Answer:
Answer:
| Physical Quantity | SI Unit | Symbol |
|---|---|---|
| i. Potential Difference | Volt | V |
| ii. Electrical Resistance | Ohm | Ω |
| iii. Temperature | Kelvin | K |
| iv. Amount of Substance | Mole | mol |
Answer:
| Fundamental Units | Derived Units |
|---|---|
| second (s) | joule (J) |
| Kelvin (K) | watt (W) |
| mole (mol) | hertz (Hz) |
| Pascal (Pa) | |
| Newton (N) |
5. Long answer type questions.
a. Explain the functions of variables in a scientific experiment.
Answer:
i. Independent Variable
Function: It is the variable that the experimenter changes or manipulates to observe its effect.
Purpose: To test its impact on the dependent variable.
Example: Changing the length of a ramp (effort distance) in an experiment.
ii. Dependent Variable
Function: It is the variable that is measured or observed during the experiment.
Purpose: To see how it responds to changes in the independent variable.
Example: Measuring the amount of force (effort) needed to move a load.
iii. Controlled Variables (Constants)
Function: These are variables that are kept the same throughout the experiment.
Purpose: To ensure a fair test and isolate the effect of the independent variable.
Example: Keeping the weight of the load and the surface material constant.
iv. Extraneous Variables (optional mention)
Function: These are unwanted variables that might affect the results if not controlled.
Purpose: To minimize error and improve experimental accuracy.
Example: Friction, air resistance, or temperature changes.
b. Enlist the characteristics of standard units.
Answer:
i. They are Universally Accepted - A standard unit must be accepted and used globally to ensure consistency in measurements.
ii. They are Well-Defined - It should have a clear and unambiguous definition, ensuring that the unit can be precisely and accurately reproduced.
iv. They are easily Reproducible - It should be possible to reproduce the standard unit easily and accurately anywhere in the world.
v. They are convenient in Size - The unit should be of a magnitude that is practical for everyday use and scientific work (not too large or too small).
vi. They are Independent of Physical Objects (Ideally) - Modern standard units should be based on fundamental constants of nature rather than physical artifacts, to avoid variability.
vii. They are compatible with Derived Units - The standard units should support the derivation of other units (e.g., Newton for force, derived from kilogram, meter, and second).
c. What are the fundamental units involved in the following derived units?
iv. Watt
Answer:
d. Why are dimensional equations important in the field of science?
Answer:
Dimensional equations are important in the field of science because they help us to understand the relationships between different physical quantities. By analyzing the dimensions (such as length, time, mass, etc.) of various physical quantities involved in an equation, we can check the consistency of the equation and verify if the derived formula is correct. This helps in avoiding errors and ensures accuracy in scientific calculations and theories.
e. What are the limitation of unit-wise analysis of physical equation in science?
Answer:
ii. Only applicable to dimensionally homogeneous equations - It can only be applied to equations that are dimensionally homogeneous, meaning all terms must have the same dimensions. It can't be used to analyze equations that mix dimensionless and dimensional quantities improperly.
iii. Fails for trigonometric, exponential, or logarithmic functions - Dimensional analysis does not work when dealing with functions like sin(θ), e^x, or log(x), because their arguments must be dimensionless, and the method can’t handle these forms properly.
iv. Cannot distinguish between vector and scalar quantities - Unit-wise analysis does not differentiate between vector and scalar quantities, even though their physical meanings and behaviors are different.
v. Cannot predict functional form accurately - Although it can suggest a possible relationship between variables, it cannot provide the exact form of the equation - such as whether it's linear, quadratic, or involves higher powers.
vi. Inapplicable to empirical or experimentally derived laws - Some physical laws are purely empirical (e.g., Ohm’s Law or Boyle’s Law), and dimensional analysis cannot derive or verify them from first principles.
Answer:
Unit-wise (or dimensional) analysis of physical equations involves checking if the units on both sides of an equation are consistent. While this method is useful for catching simple errors, it has limitations. One limitation is that it does not account for the numerical values or coefficients in the equation. Secondly, it may overlook more complex dimensional inconsistencies that could lead to incorrect conclusions. Thirdly, it cannot distinguish between scalar and vector quantity. Fourthly, it does not provide extra details about the constant in the equation. And also it fails for trigonometric, exponential or logarithmic functions. Therefore, relying solely on unit-wise analysis without considering the actual dimensions of the quantities involved can be misleading.
f. Using the principle of homogeneity, evaluate the accuracy of the physical equation s = ut +1/2 at². In the equation, s stands for displacement, u for initial velocity, t for time, and a for acceleration.
Answer:
g. Hari conducted an experiment to see how catalysts A, B and C affect chemical reaction rates. He used the same reactants but tested at different temperatures for a fixed time. Answer the following questions based on the experiment.
Answers:
Controlled (constant) variables - The reactants used, the reaction time, Other conditions (e.g., pressure, volume, concentration - if not intentionally varied).
Experimental condition that varied additionally - Temperature, which appears to be intentionally changed for further analysis.
Answer: Different temperatures were likely used to study how temperature affects the activity of each catalyst. Since catalysts often have temperature-dependent performance, Hari might have wanted to find:
- The optimal temperature for each catalyst.
- How each catalyst behaves under different thermal conditions.
This helps to provide a more complete understanding of each catalyst's effectiveness.
h. Raman wants to investigate which colour of an object has the highest capacity to absorb heat. He took four tin cans and painted them with black, white, green and red colours respectively. He poured an equal amount of water into each can, closed the openings, and placed them in sunlight for an hour. After that, he measured the temperature of the water in each can using a thermometer. Answer the following questions based on the experiment.
Answer: Below are the answers to the questions based on Raman's heat absorption experiment:-
☄ The colour of the tin cans (black, white, green, red) - Independent Variable.
☄ The temperature of the water in each can after exposure to sunlight - Dependent Variable.
ii. Which variables should be controlled by Raman? Explain.
☄ Amount of water in each can – should be equal to ensure consistent heat capacity.
☄ Type and size of the cans – identical material and dimensions so the only difference is the colour.
☄ Exposure to sunlight – place all cans in the same location to receive equal sunlight.
☄ Duration of exposure – all cans must remain in the sun for exactly the same time (1 hour).
☄ Initial temperature of water – should be the same in each can before starting the experiment.
☄ Lid or cover – use similar lids to prevent heat loss and ensure uniform conditions.
These controls help ensure that only the colour is influencing the temperature change.
iii. What is the hypothesis of this experiment?
iv. How will Raman ensure that the temperature measurements are accurate?
☄ Using a reliable and calibrated thermometer for each reading.
☄ Measuring immediately after removing the cans from sunlight to avoid heat loss.
☄ Inserting the thermometer to the same depth in each can.
☄ Taking multiple readings for each can and calculating the average.
☄ Ensuring the thermometer is not touching the sides or bottom of the can, which may affect the reading.
v. How might Raman extend this experiment to test other factors that affect the absorption of heat by an object?
☄ Test different materials of cans (e.g., aluminum, plastic, glass) while keeping the colour constant.
☄ Vary the surface texture (e.g., matte vs. glossy) to see if that influences absorption.
☄ Change the time of exposure (e.g., 30 mins, 2 hours) to analyze rate of temperature increase.
☄ Use different light sources (e.g., artificial lamps vs. natural sunlight).
☄ Compare open vs. closed cans to study heat loss.
☄ Add insulating materials to test their effectiveness in preventing heat gain.
i. Why are independent and dependent variables important in scientific study?
Answer:
1. Clarify Cause-and-Effect Relationships:
☄ The independent variable is what the scientist changes or controls.
☄ The dependent variable is what is measured or observed.
☄ This setup helps scientists determine whether changes in the independent variable cause changes in the dependent variable.
2. Guide Experimental Design:
☄ Identifying variables helps researchers:
☄ Set up a controlled and focused experiment
☄ Decide what to measure and how to measure it
☄ Keep other factors constant to ensure valid results
3. Enable Data Collection and Analysis:
☄ Knowing which variable is dependent allows for proper data tracking and comparison.
☄ Data can be visualized in graphs (e.g., dependent variable on the y-axis vs. independent variable on the x-axis) to observe trends.
4. Support Reproducibility:
☄ Clear identification of variables helps other scientists replicate the experiment and verify the findings.
☄ This is critical for building reliable scientific knowledge.
5. Test Hypotheses:
☄ Hypotheses are usually framed as predictions about how changing one variable (independent) will affect another (dependent).
☄ Accurate identification of these variables is essential to scientific reasoning and hypothesis testing.
In summary, Independent and dependent variables are key tools for Understanding relationships, Designing sound experiments, Collecting accurate data, Reaching valid conclusions, etc. They form the backbone of scientific inquiry.
j. Can you include more than one independent and dependent variable in scientific study?
Answer: Yes, we can include more than one independent and dependent variable in scientific study but it requires careful planning to ensure the results are valid and interpretable. Multiple independent variables allow researchers to explore the combined or interacting effects of different factors on an outcome, while multiple dependent variables allow for a more comprehensive understanding of the outcome by measuring various facets of it.
Researchers may include more than one independent variable to study:
☄ How each factor individually affects the outcome?
☄ How the variables interact with each other?
Example:
Studying plant growth with:
☄ Amount of sunlight (first independent variable)
☄ Type of fertilizer (second independent variable)
This setup allows you to explore:
☄ The effect of each variable on growth
☄ Whether the combination of sunlight and fertilizer has a unique effect
This type of design is called a multifactorial or factorial experiment.
2. Multiple Dependent Variables:
A study can also have more than one dependent variable, especially if you're interested in observing several outcomes.
Example:
In a health study testing a new drug, dependent variables could include:
☄ Blood pressure
☄ Heart rate
☄ Cholesterol level
Each of these outcomes provides a different way to evaluate the drug’s effectiveness.
☄ Complexity increases: Managing multiple variables can make the experiment and data analysis more complex.
☄ Control is essential: You must carefully control other variables to isolate the effects of your independent variables.
☄ Statistical analysis: Advanced statistical tools (e.g., ANOVA, regression) are often needed to analyze the effects accurately.
k. Proof that:
Answer:
Current I is in amperes (A)
1 volt (V) is defined as:
Answers:
u: initial velocity → unit = m/s
t: time → unit = s
a: acceleration → unit = m/s²
m. Controlled variables are important in scientific learning. Why?
Answer: Controlled variables are important in scientific learning because they ensure that an experiment is fair, reliable, and focused. Here is why they are important:
1. Ensure a Fair Test:☄ Controlled variables are the factors that are kept constant throughout an experiment.
☄ By controlling these, you make sure that the only thing affecting the outcome is the independent variable.
☄ This allows you to accurately test cause and effect.
2. Increase Accuracy and Reliability:
☄ Controlling variables reduces errors and variability in results.
☄ This makes your experiment more precise and repeatable by others.
3. Avoid Confounding Factors:
☄ If multiple variables change at once, it’s unclear which one caused the observed result.
☄ Controlled variables eliminate these confounding factors, keeping the experiment focused and meaningful.
4. Support Valid Conclusions:
☄ Only by keeping other variables constant you can confidently say that the independent variable caused the change in the dependent variable.
☄ This leads to more credible scientific conclusions.
n. Why can independent variables be manipulated but not dependent variables?
Answer: Independent variables can be manipulated because they are the "cause" in an experiment, while dependent variables cannot be manipulated because they are the "effect."
1. Independent Variable = What You Change:☄ The independent variable is under the control of the experimenter.
☄ You manipulate it on purpose to see how it affects something else.
Example: In an experiment to see how light affects plant growth, the amount of light is the independent variable.
You choose to expose one plant to 2 hours of light, another to 4, and another to 6.
2. Dependent Variable = What You Measure:
☄ The dependent variable is what you observe or measure in response to the changes you made to the independent variable.
☄ You do not manipulate it — it changes on its own based on what you did to the independent variable.
Continuing the example: You measure the height of each plant. That’s your dependent variable.
You don’t control how tall they grow — you just record the result.
⚠️ Why not manipulate the dependent variable?
🔖Because the experiment wouldn’t test anything!
☄ If you manually adjust or influence the dependent variable, it no longer reflects the effect of the independent variable.
☄ You can’t learn about cause and effect if you interfere with the outcome you're trying to observe.
o. Why is it important for a scientist to communicate the results and conclusions of a study?
Answer: It is very important for a scientist to communicate the results and conclusions of a study because this is how science advances, becomes useful, and stays credible. Here are the main reasons:
1. Allows Others to Verify the Findings:☄ When scientists share their methods and results, others can repeat the experiment to confirm its accuracy.
☄ This process is called replication, and it helps build trust and reliability in scientific knowledge.
2. Contributes to the Body of Knowledge:
☄ Science is a collaborative effort.
☄ By sharing findings, a scientist adds to what is already known, helping others build on their work or avoid repeating the same experiment.
3. Encourages Peer Review and Feedback:
☄ Communicating results through papers, presentations, or reports allows other experts to evaluate the work.
☄ Peer review helps catch mistakes, improve quality, and ensure the conclusions are supported by evidence.
4. Solves Real-World Problems:
☄ Scientific research often leads to solutions in medicine, engineering, agriculture, technology, and more.
☄ If results aren't communicated, society misses out on important discoveries and innovations.
5. Informs Policy and Public Decisions:
☄ Scientific findings guide decisions about health, environment, safety, education, and many other areas.
☄ Sharing results ensures that governments, organizations, and the public make choices based on solid evidence.
6. Inspires Further Questions and Research
☄ Every experiment opens the door to new questions.
☄ Sharing results helps other scientists identify new areas to explore, leading to continuous progress.
6. Diagrammatic questions:
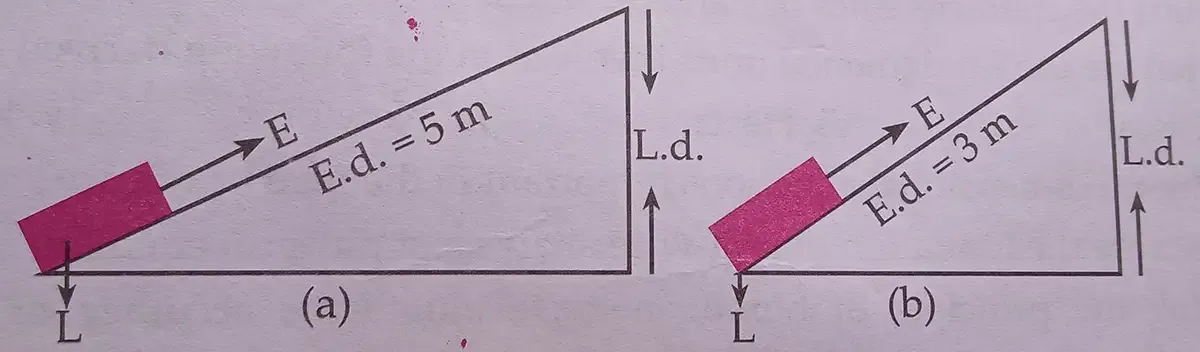 |
| Experiment - Effect of effort distance of the slanted surface on the effort applied to lift up the load. |
i. What variables are involved in it?
Answer: The variables involved in it are -
ii. Which variables have to be controlled in the experiment?
Answer: The following variables have to be controlled in the experiment -
(a) Load (L) – same weight in both setups.(b) Load Distance (L.d.) – same vertical height in both cases.
(c) Surface type and friction – use the same material and maintain conditions.
(d) Inclined plane width – same size platform.
(e) Measurement tools – same force meter, etc.
iii. Sort out independent, dependent and combined variables in it.
Answer:
Extra Tips: ⛄
Fundamental Units (Basic Units) and Dimensions of Fundamental Units.
| Physical Quantity | Unit (SI) | Dimension Symbol |
|---|---|---|
| Length | meter (m) | L |
| Mass | kilogram (kg) | M |
| Time | second (s) | T |
| Temperature | kelvin (K) | Θ (Theta) |
| Electric Current | ampere (A) | I |
| Luminous Intensity | candela (cd) | J |
| Amount of Substance | mole (mol) | N |
Derived Units and their relation with Fundamental Units.
| S.N. | Derived Quantity | Related Formulae | Symbol of Unit Name | Derived Units | Fundamental Units Involved |
|---|---|---|---|---|---|
| 1. | Area (A) | length×breadth (l×b) | square metre | m² | m×m |
| 2. | Volume (V) | length×breadth×height (l×b×h) | cubic metre | m³ | m×m×m |
| 3. | Velocity (v) | displacement/time (s/t) | metre per second | ms⁻¹ | m/s |
| 4. | Acceleration (a) | change in velocity/time (v-u/t) | metre per square second | ms⁻² | m/(s×s) |
| 5. | Force (F) | mass×acceleration (m×a) | kilogram×metre per square second or Newton | N | kg×m/(s×s) |
| 6. | Density (or d) | mass/volume (m/v) | kilogram per cubic metre | kg/m³ or kgm⁻³ | kg/(m×m×m) |
| 7. | Pressure (P) | force/area (F/A) | Newton per square metre or Pascal | Pa or Nm⁻² | kg/(m×s×s) |
| 8. | Momentum (p) | mass×velocity (m×v) | kilogram×metre per second | kgms⁻¹ | kg×m/s |
| 9. | Work and Energy (W/E) | force×displacement (F×s) | Newton×metre or joule | Nm or J | (kg×m×m)/(s×s) |
| 10. | Power (P) | workdone/time (w/t) | Joule per second or watt | Js⁻¹ or W | (kg×m×m)/(s×s×s) |
| 11. | Frequency (f) | velocity/wavelength (v/λ) | 1/second or Hertz | Hz | cycle/s or s⁻¹ |
| 12. | Potential Difference (V) | work done/charge (w/q) | newton×metre/Columb (second×Ampere) or volt (J/C) | V | (kg×m×m)/(s×s×s×A) |
| 13. | Resistance (R) | potential difference/current (V/I) | Newton×metre/columb×second×Ampere Or ohm | Ω | (kg×m×m)/(s×s×s×A×A) |
6. The unit of Area is a derived unit. Justify.
Mathematically, for a rectangle, Area = length × width.
Both length and width are measured in the base unit of metres (m) in the SI system.
Therefore,
Since m² is formed by combining the base unit metre (m), the unit of Area is derived unit.
7. The unit of Volume is a derived unit. Justify.
For a cube or rectangular prism,
Each of these dimensions is measured in the base unit of length, which is the metre (m) in the SI system.
So, the unit of volume becomes:
8. The unit of Velocity is a derived unit. Justify.
In the SI system:
Displacement is measured in metres (m) → a base unit of length.
Time is measured in seconds (s) → a base unit of time.
So,
9. The unit of Acceleration is a derived unit. Justify.
So, the unit of acceleration becomes: m/s ÷ s = m/s² = ms⁻²
10. The unit of Force is a derived unit. Justify.
Answer:
11. The unit of Density is a derived unit. Justify.
12. The unit of Pressure is a derived unit. Justify.
The above Unit 1 Scientific Learning Class 10 Science and Technology Guide/note could be a valuable resource for both teachers and students.
Benefits for Teachers:
Structured Teaching: Provides a clear lesson structure, making teaching systematic and effective.
Key Points: Highlights important concepts to cover, ensuring comprehensive teaching.
Engaging Activities: Includes practical activities and experiments to make learning interactive.Benefits for Students:
Easy Understanding: Simplifies complex concepts, making them easier to grasp.
Note-Making: Helps in creating organized and effective notes.
Exam Preparation: Offers practice questions and revision tips useful for terminal exams and SEE (Secondary Education Examination).Importance of Using This Guide:
1. Clear Explanations: Breaks down difficult topics into understandable parts.
2. Consistent Learning: Ensures teachers and students are aligned on learning objectives.
3. Confidence Building: Regular practice and revision build exam confidence.
4. Real-Life Applications: Connects scientific concepts to daily life, enhancing interest and relevance.(alert-passed)


First of all, thank you for taking the time to read my blog. It's much appreciated! If you would like to leave a comment, please do, I'd love to hear what you think!
Suggestions and/or questions are always welcome, either post them in the comment form or send me an email at drgurung82@gmail.com.
However, comments are always reviewed and it may take some time to appear. Always keep in mind "URL without nofollow tag" will consider as a spam. 😜